EpiPen®(adrenaline) Auto-Injector is indicated in the emergency treatment of severe allergic reactions (anaphylaxis) to insect stings or bites, foods, drugs and other allergens, as well as idiopathic or exercise induced anaphylaxis.1,2
- It delivers the correct dose of adrenaline quickly. Each auto-injector contains a single dose of adrenaline.1,2
- It has needle protection which deploys immediately after activation.1,2
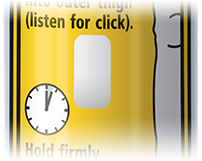
- It is designed for easy use and should simply be jabbed firmly against the outer portion of the thigh from a distance of approximately 10 cm. When jabbed against the thigh, it releases a spring-activated plunger, pushing concealed needle into the thigh muscle and expelling a dose of adrenaline.1,2
| EpiPen® Auto-Injector | Adrenaline concentration | Body weight |
|---|---|---|
| EpiPen® 300 micrograms solution for injection in pre-filled pen1 | 0.3 mg (per 0.3 ml) | >30kg (4 st. 10 lbs.) |
| EpiPen® Junior 150 micrograms solution for injection in pre-filled pen2 | 0.15 mg (per 0.3 ml) | 15-30kg ( 2 st. 5 lbs. - 4 st. 10 lbs.) |
mg = milligram; ml = millilitre; kg = kilogram; st. = stone; lbs = pounds
In children below 15 kg in weight: The suitability of EpiPen® Junior has to be judged individually. The use in children weighing less than 7.5 kg is not recommended unless in a life-threatening situation and under medical advice.1,2
Features
How to use - instructions for patients1,2
In this context, EpiPen® auto-injector refers to both EpiPen® and EpiPenJunior®.
1. Grasp EpiPen® auto-injector in dominant hand (the hand you use to write), with thumb nearest blue cap and form fist around the unit (orange tip down).
3. Hold the EpiPen® auto-injector approximately 10 cm away from the outer thigh, with the orange tip pointing towards the outer thigh.
5. Hold firmly against thigh for 3 seconds. The injection is now complete and the viewing window on the Auto-Injector is obscured.
6. EpiPen® should be removed (the orange needle cover will extend to cover needle) and safely discarded. Gently massage the injection area for 10 seconds. Dial 112, ask for ambulance, state "anaphylaxis" even if symptoms appear to be improving.
EpiPen® Trainer Pen
For training and demonstration purposes, an EpiPen® training device (without drug, without needle) is available.
The label clearly states EpiPen® Trainer and it is pale blue and grey in colour. The training device and the active EpiPen® Auto-Injector should not be routinely carried together in order to avoid confusion in an emergency situation.
The Epipen® Trainer is reusable so patients can practice as many times as they like. It works in the same way as the EpiPen auto-injector but doesn't contain a needle or any adrenaline.3,4
When to use
Anaphylaxis is defined as a severe life-threatening generalized or systemic hypersensitivity reaction characterized by rapidly developing airway and/or circulation problems.5
A person who is having an allergic reaction should use their EpiPen® immediately if they experience ANY of the following serious symptoms of anaphylaxis following contact with their allergen:6
- Persistent cough
- Hoarse voice
- Difficulty swallowing
- Swollen tongue
- Difficult or noisy breathing
- Wheeze or persistent cough
- Persistent dizziness
- Pale or floppy
- Suddenly sleepy
- Collapse/unconscious
Other allergy symptoms may include an itchy, raised rash (hives), feeling or being sick, swelling (angioedema) or stomach pain.3,4
Patients experiencing the following mild to moderate symptoms of allergy should take an anti-histamine and have their EpiPen® ready in case of worsening symptoms:6
- Red, raised, itchy rash
- Swelling of face, lips or eyelids
- Abdominal pain, nausea and/or vomiting
- Tingling mouth
In the absence of clinical improvement or if deterioration occurs, a second injection with an additional EpiPen® Auto-Injector may be administered 5 - 15 minutes after the first injection. It is recommended that patients are prescribed two EpiPen® pens which they should carry at all times.1,2
In this context, EpiPen® auto-injector refers to both EpiPen® and EpiPenJunior®.
- EpiPen Auto-Injectors are intended for immediate administration in patients, who are determined to be at increased risk for anaphylaxis, including individuals with a history of anaphylactic reactions.1,2
- The healthcare professional prescribing an EpiPen® Auto-Injector must ensure that the patient understands the indications for use and the correct method of application. Therefore, the healthcare professional should discuss the contents of the patient information leaflet, the correct handling of the Auto-Injector and the possible symptoms of an anaphylactic episode in detail with the patient.3,4
- In case support is needed in the emergency situation, it is strongly advised to also educate the patient's family, friends, carers, teachers etc.3,4
Safety information
If used correctly, significant injury with EpiPen® is unlikely. Patients should not remove the blue safety cap until they are ready to inject, always ensure that they point the end with the orange tip towards the thigh and never put their thumbs or fingers over the orange tip. Directions for use are in the package insert.1,2
Potential side effects include an increase in heart rate, a stronger or irregular heartbeat, sweating, nausea and vomiting, difficulty breathing, paleness, dizziness, weakness or shakiness, headache, apprehension, nervousness, or anxiety. Adrenaline is ordinarily administered with extreme caution to patients who have a heart disease. Adrenaline should only be prescribed to those patients, but also those suffering from diabetes, hyperthyroidism, hypertension and elderly individuals if the potential benefit justifies the potential risk. There is a risk of adverse reactions following epinephrine administration in patients with high intraocular pressure, severe renal impairment, prostatic adenoma leading to residual urine, hypercalcaemia and hypokalaemia. In patients with Parkinson's disease, epinephrine may be associated with a transient worsening of Parkinson symptoms such as rigidity and tremor.1,2
Patients with concomitant asthma may be at increased risk of a severe anaphylactic reaction.1,2
Adrenaline inhibits the secretion of insulin, thus increasing the blood glucose level. It may be necessary for diabetic patients receiving adrenaline to increase their dosage of insulin or oral hypoglycaemic drugs.1,2
It is important to remember that in practice there are no known absolute contraindications to the use of adrenaline in a in a life-threatening allergic reaction.1,2
Overdose or inadvertent intravascular injection of adrenaline may cause cerebral haemorrhage resulting from a sharp rise in blood pressure. Fatalities may also result from pulmonary oedema because of peripheral vascular constriction together with cardiac stimulation. Pulmonary oedema may be treated with α-blocking agents such as phentolamine. In case of arrhythmias these may be treated with ß-blocking agents.1,2
If you have any concerns/questions about the safety aspects of EpiPen®, please contact the Viatris Ireland Medical Information Department on +353 (0)1 8711600 (option 1) or email: info.ie@viatris.com
For the full details of side effects, warnings and precautions, please refer to EpiPen® or EpiPen® Junior Summary of Product Characteristics (SmPC)
- EpiPen (adrenaline) 300 micrograms solution for injection in pre-filled pen, Summary of Product Characteristics. Available at: www.medicines.ie. Last accessed: 24th October 2025.
- EpiPen Junior (adrenaline) 150 micrograms solution for injection in pre-filled pen, Summary of Product Characteristics. Available at: www.medicines.ie. Last accessed: 24th October 2025.
- EpiPen (adrenaline) 300 micrograms solution for injection in pre-filled pen, Patient Information Leaflet (PIL) Available at: www.medicines.ie. Last accessed: 24th October 2025.
- EpiPen Junior (adrenaline) 150 micrograms solution for injection in pre-filled pen, Patient Information Leaflet (PIL). Available at: www.medicines.ie. Last accessed: 24th October 2025.
- Alvarez-Perea, A., Tanno, L.K. & Baeza, M.L. How to manage anaphylaxis in primary care. Clin Transl Allergy 7, 45 (2017). https://doi.org/10.1186/s13601-017-0182-7
- Muraro A, et al. EAACI guidelines: Anaphylaxis. 2022;77: 357-377
Register for Viatris Connect today ?
Please note that the website contains promotional and non-promotional materials, including educational content and resources to help you and your patients.
REGISTER NOW